Making Excellence Easier
Softneta: Pioneering Innovation in Medical Imaging Solutions
.
Selecting Arazy Group: A Strategic Move for Softneta's Growth
In 2015, securing venture capital funding marked a turning point for the company, necessitating Class 2 medical device certification. Softneta's expansion plans extended beyond Europe to include the US market. Their venture capital partner, aware of their needs, suggested exploring potential partners. Arazy Group emerged as a strong contender, known for its expertise and early successes. Despite Arazy Group not being the most economical option presented during the tender process, "we chose them for their stellar reputation, endorsed by our investor and my personal experiences." This decision led to a successful long-term collaboration, culminating in the timely acquisition of FDA approval.
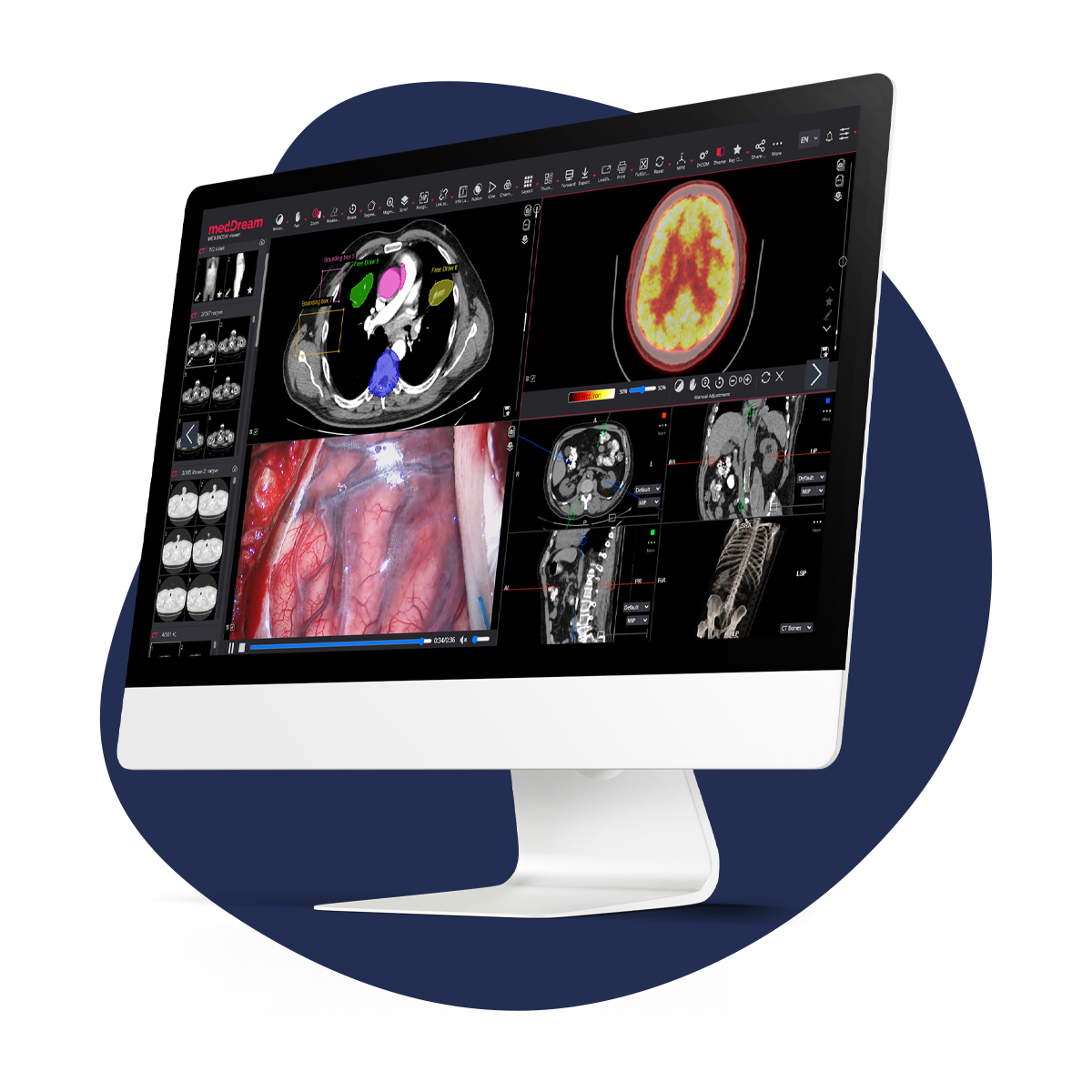
At this point, Softneta was introduced to Ray Kelly, Arazy Group's not-so-secret weapon for ensuring FDA approval. Recently, Ray, an accomplished regulatory professional, has achieved a significant milestone in his career by obtaining FDA clearance for over 100 traditional 510(k) notifications for innovative medical products. His spectacular results stem from his core beliefs about the regulatory profession. "Sometimes we get caught up in the daily work challenges and forget that the main mission for every regulatory professional... still is to help patients and improve their lives; we should never forget this." In 2016, Ray shared that they had introduced the MedDream Medical Image Management and Processing System. Softneta, comprised of industry professionals, proved to be an excellent collaborator. Arazy Group swiftly secured FDA approval for their SaMD. After learning about their interest in adding mammographic images and biosignals, Ray promptly assisted with a second successful FDA submission.
"In the spirit of fostering the valuable partnership, we're currently aiding Softneta in obtaining MDSAP certification and software licensing in Canada." - Ray Kelly
Overcoming Obstacles
.Their Quality Documentation Manager oversees this process, which involves eight to nine individuals within the quality management system. This includes developers, support staff, test personnel, analytical experts, managers, and department heads.
Looking Into the Future
.As a Chief Technology Officer, Tomas shared his predictions regarding how AI will change this industry over the next decade. He envisions AI playing a pivotal role. Ideally, it will tackle the most complex processing tasks, especially in medical workstations where doctors face intricate decision-making processes. The current focus is on simpler diagnostic tasks, leaving out the full diagnostic spectrum. However, he anticipates AI covering these complex functions, providing reports and straightforward visualizations for doctors to swiftly comprehend without the need for manual manipulation.
Everyone collaborates to ensure compliance with documentation standards for their product releases, which occur three to four times annually. Each release requires significant documentation updates, which can take several weeks, making agile release schedules challenging.
Despite these constraints, they've adapted and consistently managed three to four releases per year over the last six years, with the quality of releases steadily improving. This consistency enables us to maintain secure documentation and swiftly address any issues.
Partnering with reliable suppliers like Arazy Group has also been essential. The support ensures that any challenges encountered during the certification process are effectively managed, leading to timely certification and, ultimately, benefiting patient health.
Softneta has experienced much success during their exponential growth. One story of note was that Swisscom, a significant telecom entity, was servicing 160 hospitals, marking an important milestone for the local and global market. They emphasized the importance of Swiss-level quality in both code and product security. This prompted the company to undertake significant cybersecurity enhancements. Despite the challenges, they successfully met Swisscom's standards and became a supplier for the telecom giant. Softneta takes pride in being Swisscom's solution provider and in how this partnership drove positive changes in their product, particularly cybersecurity.

Moreover, AI's integration into various industries, including regulatory affairs, may lead to the displacement of specific jobs. While highly skilled professionals will still be in demand, there will be a shift towards more specialized roles, leaving a gap for those needing to upskill. Balancing the utilization of AI with the retention of skilled human labour presents a significant challenge for the industry.
Tomas Dumbliauskas finds great fulfillment in his career by observing his team's accomplishments surpass his expectations. He highlights the joy of witnessing Softneta employees excel in tasks or challenges where he may have limitations. For him, the strength of a company lies in its diverse team, each contributing unique expertise and experiences. Although such moments may not occur daily, they provide immense satisfaction, reinforcing his confidence in the team's capabilities and the company's success.
Softneta, a company dedicated to supporting startups, particularly in the AI sector, offers their solutions free of charge during the development phase, understanding the financial constraints early-stage ventures face. Softneta facilitates a seamless integration experience for startups by ensuring compatibility with AI solutions. Softneta often recommends Arazy Group to these companies for specialized support in certification procedures, highlighting their commitment to aiding emerging ventures in overcoming early hurdles. A full circle of support from the early days when Benny extended his expertise.
For emerging MedTech companies seeking accelerated growth and risk mitigation, partnering with Arazy Group provides an unparalleled opportunity. With a proven track record of success and expertise in navigating complex regulatory landscapes, Arazy Group empowers companies to navigate certification processes seamlessly and confidently expand their global market reach.
If you are curious about how you can expand your global reach most cost-effectively, please get in touch with us at Arazy Group.
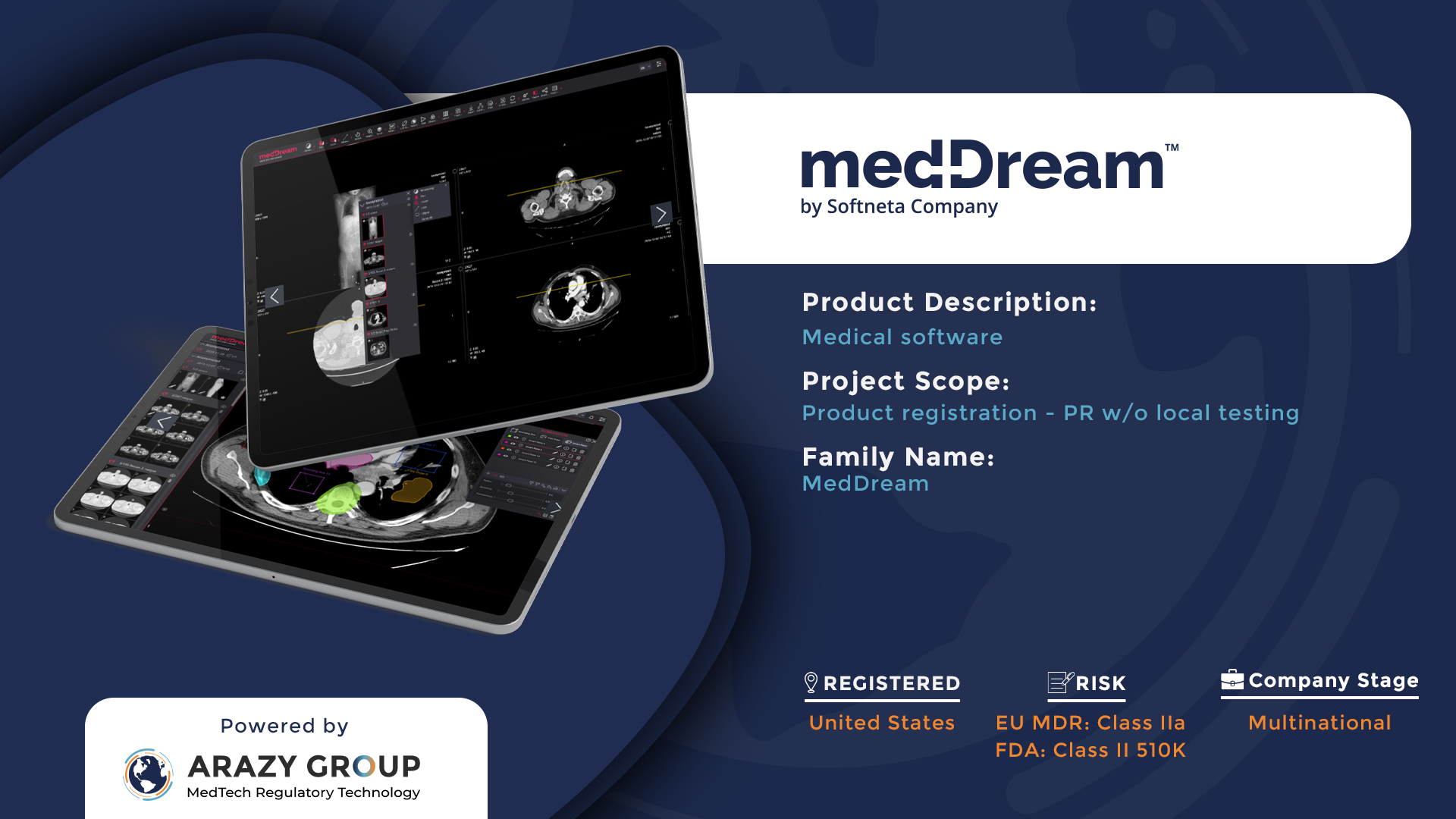
Softneta Products Registered in Multiple Countries
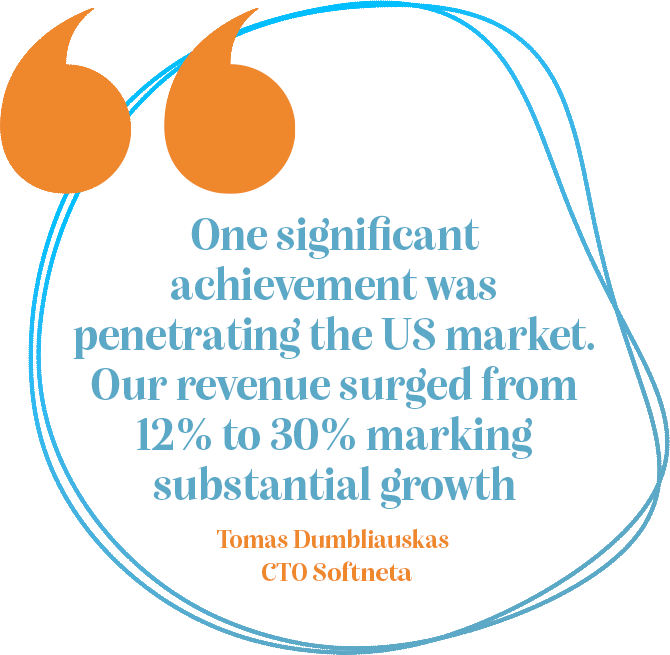
MAKING THE BEST MEDICAL DEVICES AND PRACTICES ACCESSIBLE TO PATIENTS WORLDWIDE AS SOON AS POSSIBLE...
Our approach to achieving this critical goal is by providing a single platform that offers all necessary safety compliance information, registration management tools, and expert support to each function within an organization’s regulatory affairs department and to the other stakeholders involved in bringing products to market.