The world of medical technology is transforming, not just in innovation but also in how these...

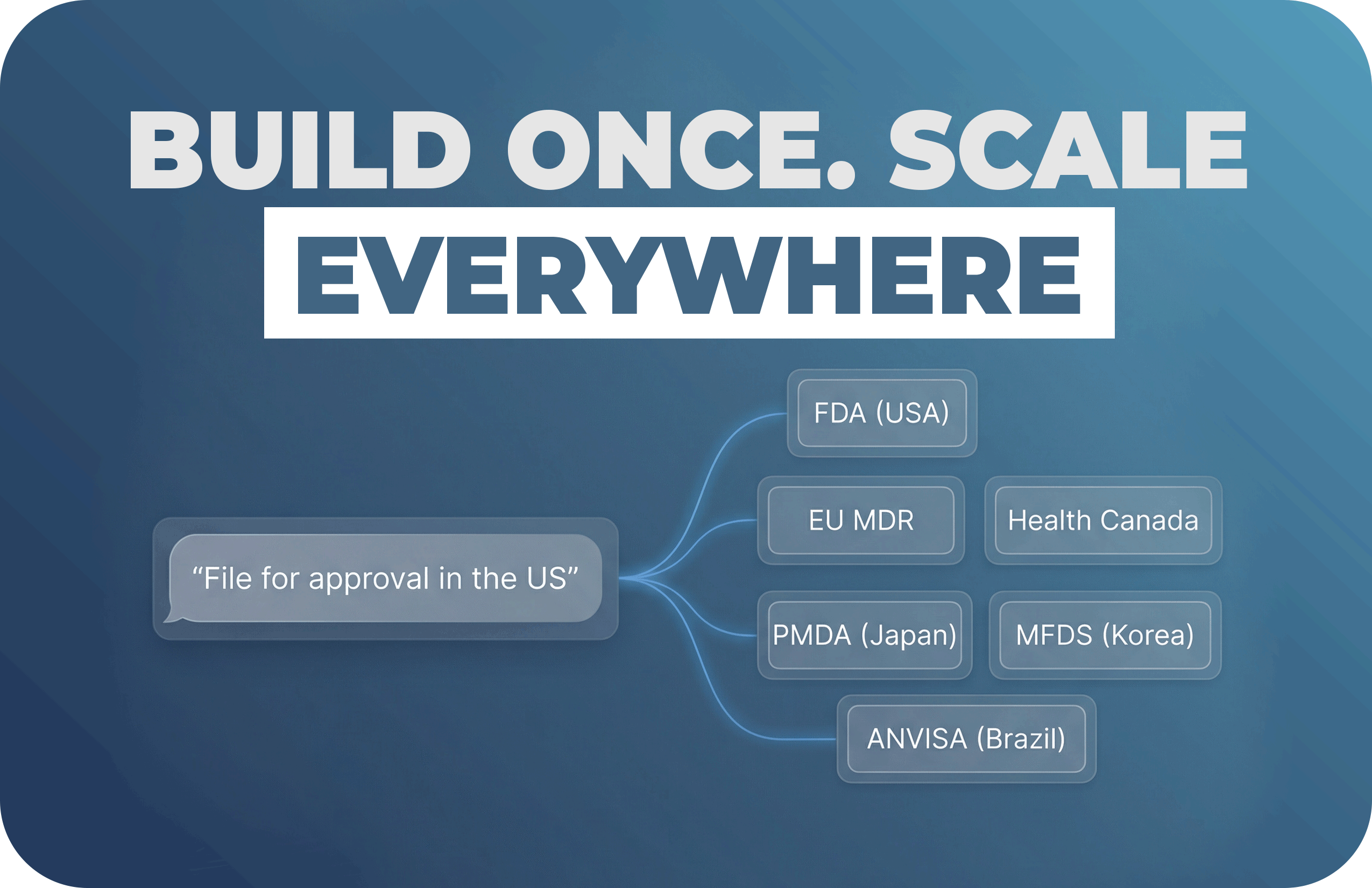
A straightforward plan for teams that want real progress, not another “AI experiment.”

How a Small RA Team Should Measure AI Impact, Choose the Right Level of Adoption, and Navigate...

…. And what they mean for your 2026 budget



Artificial Intelligence (AI) has quickly become one of the hottest topics in MedTech. Today’s...

Strengthening Compliance: Taiwan Releases Preclinical Testing Standards for Key Medical Devices
.png)
Here are essential deadlines, new post-market surveillance rules, and compliance strategies for...

Accelerating Innovation: Saudi Arabia’s Fast Track Pathways for Innovative Medical Devices